Background: Outcomes for children with relapsed acute myeloid leukemia (AML) and subgroups with relapsed acute lymphoblastic leukemia (ALL) remain poor, and therapeutic options are limited. There is no standard of care for relapsed disease, and data collection after relapse is lacking. In recent years, extensive molecular studies of pediatric leukemias have advanced target identification and clinical trial development. Such studies have shown that pediatric leukemias are biologically distinct from adult leukemias. The current paradigm of requiring new agent studies in adults prior to conducting pediatric trials is thus not appropriate. However, the rarity of relapsed leukemia in children makes the conduct of randomized registration trials for new agents infeasible. Recognizing that impactful progress requires international collaboration, the Leukemia & Lymphoma Society is sponsoring the Pediatric Acute Leukemia (PedAL) Initiative in North America, Australia and New Zealand. In parallel, the European Pediatric Acute Leukemia (EuPAL) Foundation has established a registry for pediatric relapsed leukemia. The principal goal of this international PedAL/EuPAL collaboration is to establish a data-driven developmental therapeutics program for children with relapsed/refractory leukemias.
Trial Summary: A central component of the PedAL Initiative is the screening protocol APAL2020SC (NCT04726241), which serves as the entry point for associated therapeutic sub-trials. The primary aims of APAL2020SC are (1) to use clinical and biological characteristics to screen for eligibility for therapeutic sub-trials; and (2) to maintain a longitudinal data registry and biobank for relapsed leukemia. Eligible patients are <22 years old and have one of the following leukemias in first or greater relapse, or primary refractory: AML, myeloid leukemia of Down syndrome, T-ALL, mixed phenotype acute leukemia, and KMT2A-rearranged B-ALL. Additional eligible diagnoses include any B-ALL in second or greater relapse, and de novo or relapsed treatment-related AML. All leukemia specimens are profiled by multiparameter flow cytometry (MFC) at a central lab (Hematologics). Families have the option to submit biospecimens for banking. Patients with myeloid leukemia also have molecular testing through Foundation Medicine. Clinical data collected include demographics, cytomolecular features at diagnosis, prior treatment and post-relapse treatment. Patients are encouraged to submit follow-up bone marrow samples for central MFC. The MFC assay determines lineage and blast percent and quantifies expression of markers relevant to therapeutic sub-trials.
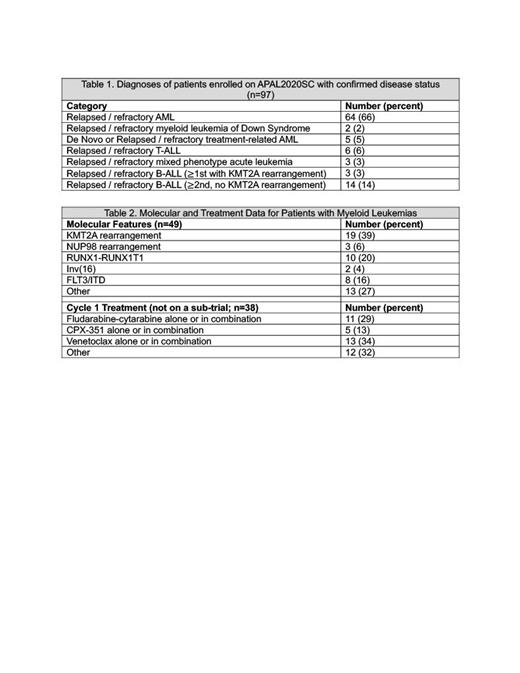
Trial Progress: APAL2020SC opened in April 2022. As of July 25, 2023, 148 patients have enrolled and 97 have had leukemia confirmed centrally. The distribution of diagnoses is shown in Table 1. All patients had blast enumeration and target expression assessed by MFC at study entry and when follow-up samples were provided. Of the patients with myeloid leukemia (n=71), targeted DNA/RNA sequencing was successful for 69%. The distribution of AML-associated alterations is shown in Table 2. Of the patients with confirmed leukemia at enrollment, 79% submitted at least 1 follow-up sample, and 77% submitted a sample for biobanking. To date, two therapeutic trials have opened that require enrollment to the screening trial. APAL2020D/ITCC101 is a randomized phase III study comparing fludarabine-cytarabine (FLA) to FLA-venetoclax. APAL2020E/PEPN2113 is a phase 1 study of the E-selectin inhibitor uproleselan in combination with FLA. Pediatric-specific therapeutic trials for menin inhibitors and other new agents are in development. The study is collecting treatment information for children who do not enroll on a subtrial, with the goal of describing current practices and outcomes (Table 2).
Conclusion: APAL2020SC will accrue patients with relapsed acute leukemias and treatment-related AML for 5 years. This protocol will promote clinical trial development by engaging industry partners and providing a single entry point to therapeutic studies with biological rationale. APAL2020SC and the EuPAL Foundation registry will generate a large, comprehensive and longitudinal dataset that will inform future trials. The biobank component will support innovative translational research to feed the pipeline of new agents.
Disclosures
O'Brien:Pfizer: Honoraria, Research Funding. Tasian:Incyte Corporation: Research Funding; Aleta Biotherapeutics: Membership on an entity's Board of Directors or advisory committees; Amgen: Other: travel support ; Beam Therapeutics: Research Funding; Kura Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Syndax Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Zwaan:Takeda: Research Funding; Pfizer: Consultancy, Research Funding; Abbvie: Research Funding; Jazz Pharmaceutical: Research Funding; Incyte: Consultancy; Novartis: Consultancy, Other: Advisory role; Kura Oncology: Consultancy; Abbvie: Research Funding; Syndax: Research Funding; Gilead: Consultancy; BMS: Consultancy; Sanofi: Other: Advisory role; ITCC Hem Malignancies Committee: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal